Early detection of Alzheimer’s disease with a simple blood test
Alzheimer's disease (AD), the leading form of dementia, casts a long shadow on millions of lives worldwide. With rising numbers of people diagnosed and new disease-modifying treatments, early detection becomes even more critical.
An important hallmark of the disease is the deposition of β-Amyloid proteins between the neurons in the brain's grey matter in the form of senile plaques. For a long time, reliable detection of β-Amyloid was only possible from CSF (cerebrospinal fluid) or using positron emission tomography (amyloid PET). Even in cases where these procedures are medically indicated, they are expensive and invasive.
Our innovative approach offers a simpler, more accessible solution: a blood test to aid in the diagnosis of elevated β-Amyloid in the brain of patients who may be at risk for Alzheimer’s Disease.
First CE-marked immunoassay for pre-screening with blood samples
On HISCL, the β-Amyloid 1-42/ 1-40 ratio is determined. Experimental results have shown that a lower plasma β-Amyloid 1-42/1-40 ratio is associated with higher cortical amyloid burden, greater cognitive decline, and therefore an increased risk of developing AD dementia. The β-Amyloid 1-42/1-40 ratio eliminates fluctuations in the total amyloid level and improves the diagnostic value compared to determining the β-Amyloid 1-42 concentration alone.
With the help of highly sensitive and fully automated procedures such as the Sysmex Automated Immunoassay System (HISCL), biomarkers for early detection of Alzheimer’s disease - such as the β-Amyloid 1-42/ 1-40 ratio - can now be measured from blood samples in just 17 minutes.
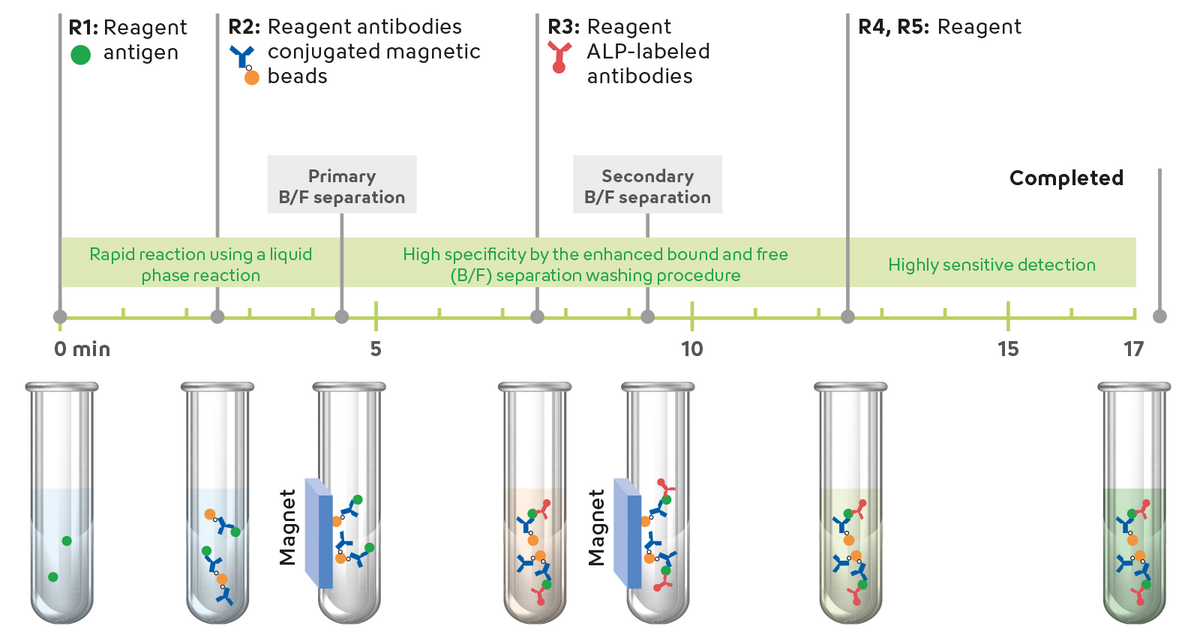
Significantly, the HISCL™ β-Amyloid 1-42 and HISCL™ β-Amyloid 1-40 assays are the first CE-marked immunoassay tests released for routine diagnosis. Additional blood-based tests are in development and will complement the range of biomarkers for detecting Alzheimer's and other neurodegenerative diseases.
High predictive accuracy for amyloid pathology
This test leverages the Sysmex Automated Immunoassay System (HISCL) to measure β-Amyloid 1-42/1-40 ratio, a key Alzheimer's biomarker, with high accuracy. Clinical studies show the HISCL β-amyloid test performs exceptionally well (AUC 0.895), rivalling more complex imaging methods like PET scans or CSF imaging.

Follow the science: Download our informative literature
Want to learn more about the groundbreaking HISCL technology and the science behind this accurate blood test? Download our informative literature to explore the details of the HISCL instrument and its role in early Alzheimer's detection.

Measurement of Aβ42/40 in plasma samples using a high throughput clinical autoanalyser is now available as an LDT to assist physicians with identifying patients with β-amyloid plaques and the potential presence of Alzheimer’s disease.

Plasma Aβ42/40 ratio measured by HISCL achieved high accuracy in predicting Aβ pathology determined by CSF testing similarly to the previous report comparing with Amyloid PET in another cohort.

Three group classification allowed our plasma Aβ assay to achieve PPV and NPV ≥ 90% with 74.4% of participants classifiable as Aβ positive or negative groups. This result indicated that our assay may contribute to reducing amyloid PET scans or CSF Aβ testing, which could be helpful in applications such as the recruitment step of clinical trials.

Our study demonstrated the utility of plasma Aβ42/40 measured using the fully automated HISCL immunoassay platform in predicting PET-derived Aβ positivity in a diverse patient population and healthy controls. It has the potential to identify early amyloid pathology. Furthermore, the equipment is widely used in clinical settings; hence, it may be used as a large-scale screening tool for the incoming era of AD-modifying therapies.

The plasma Aβ42/Aβ40 ratio measured using the HISCL series achieved high accuracy in predicting amyloid PET status. Since our blood-based immunoassay system is less invasive and more accessible than amyloid PET and cerebrospinal fluid testing, it may contribute to the diagnosis of Alzheimer’s disease in routine clinical practice.



