RNXtract® for MammaTyper®
Easy, fast and convenient FFPE sample RNA extraction kit
- Complementary CE IVD-marked product for the MammaTyper kit
- Faster procedure eliminates the need for a column system in nucleic acid purification by utilizing magnetic beads.
- Environmentally friendly and safer for users as it does not utilize a deparaffinization reagent and reduces plastic material waste.
- Fast turn-around-time, procedure is quicker and requires less steps
RNXtract® Nucleic Acid Extraction Kit
RNXtract® is intended for routine clinical use for extraction and purification of total RNA from formalin-fixed and paraffin-embedded (FFPE) sample material.
The kit offers a reliable and robust method of gaining high-quality, amplifiable template RNA from formalin-fixed, paraffin-embedded (FFPE) tissue for use in subsequent molecular biology techniques.
The kit contains eight vials of reagents that allow 48 extractions and was developed together with MammaTyper®.
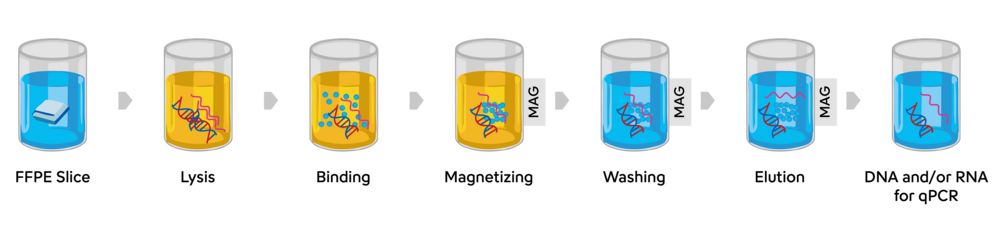
Graphical Representation of the Procedure
RNXtract® Nucleic Acid Extraction Kit overview:
- The RNXtract® Nucleic Acid Extraction Kit purifies nucleic acid from FFPE samples by using lysis buffer and proteinase K sequentially.
- The unique binding buffer system of this kit allows magnetic beads to bind nucleic acid efficiently and specifically.
- The washing system effectively removes proteins, nucleases and other impurities to provide highly purified and stable nucleic acid.
- This method does not use a deparaffinization reagent and is therefore considered friendly to the environment and safer for users.
- The method does not require plastic columns for purification, which prevents the loss of nucleic acids during purification steps.
| Feature | RNXtract® Nucleic Acid Extraction Kit |
| Sample type | 10 uM FFPE tissue section (tumor cell content >20 %) |
| Sample capacity | 48 extractions |
| Feature | MammaTyper® IVD test kit (10 reactions) |
| Sample type | 10 μm FFPE tissue section (tumor cell content > 20 %) |
| Sample capacity | Up to 8 patient samples per kit |
| QC function | 2 external controls (positive + negative) |
| Compatiple platforms | LightCycler® 480 Instrument II (Roche) Cobas z® 480 Analyzer (Roche) Versant® kPCR AD module (Siemens) Applied Biosystems® 7500 Fast (Dx) RT PCR (ThermoFisher Scientific) CFX96TM RT PCR Detection System-IVD (BIO-RAD®) MX3000P (Agilent®) Agilent® AriaDx Real-Time PCR System (Agilent®) SLAN®-96P Real-Time PCR System (Sanure Biotech) |
| Concordance | Concordance between MammaTyper® and IHC/CISH-based biomarker assessments: HER2 91.8%, ER 91.8%, PR 82.5%, KI67 75.0% 1 Overall Percentage Agreement (OPA) of assessment by MammaTyper® vs IHC/FISH (Ki67 digital image analysis): HER2 95.0%, ER 95.5%, PR 89,4%, Ki67 87,2% 4 |
| Reproducibility | Reproducibility of binary single-marker results (pos/neg), as well as the molecular subtype agreement, was almost perfect (kappa values: 0,90 – 1,00) in a 10 centre concordance study 2 |
| Reclassification | MammaTyper® may offer more precise assessment of endocrine responsiveness, improve Ki67 standardization and help resolve eqivocal HER2 IHC/FISH cases, leading to potential redistributions of the molecular subtypes 3 |
| Catalogue Number | CC01010 |
References
- Wirtz RM et al., Breast Cancer Res Treat 2016; 157(3), 437446
- Varga Z et al., Breast Cancer Res 2017; 19(55), 1-13
- Caselli E et al., PLOS ONE 2021; 16(9), 1-18
- Shaaban A et al., Eur J Cancer 2022; 175(1), 87-88
Sysmex Europe SE
Bornbarch 1
22848 Norderstedt
Germany
+49 (40) 527 26 0
+49 (40) 527 26 100